Is SARS-nCoV-2-associated systemic micro-clotting due to spike protein-induced hemolysis resulting in amyloid plaque formation?
Can Plasmodium falciparum help us answer this question?
Is SARS-nCoV-2-associated systemic micro-clotting due to spike protein-induced hemolysis resulting in amyloid plaque formation? Can Plasmodium falciparum help us answer this question?
“Recent evidence shows that SARS-CoV-2 is capable of affecting the genetics and dynamics of erythrocytes and this coexists with a non-homeostatic function of cardiovascular, respiratory and renal systems during COVID-19. In hypothesis, SARS-CoV-2-induced systematical alterations of erythrocytes dynamics would constitute a set point for COVID-19-related multiple organ failure syndrome and death.”1
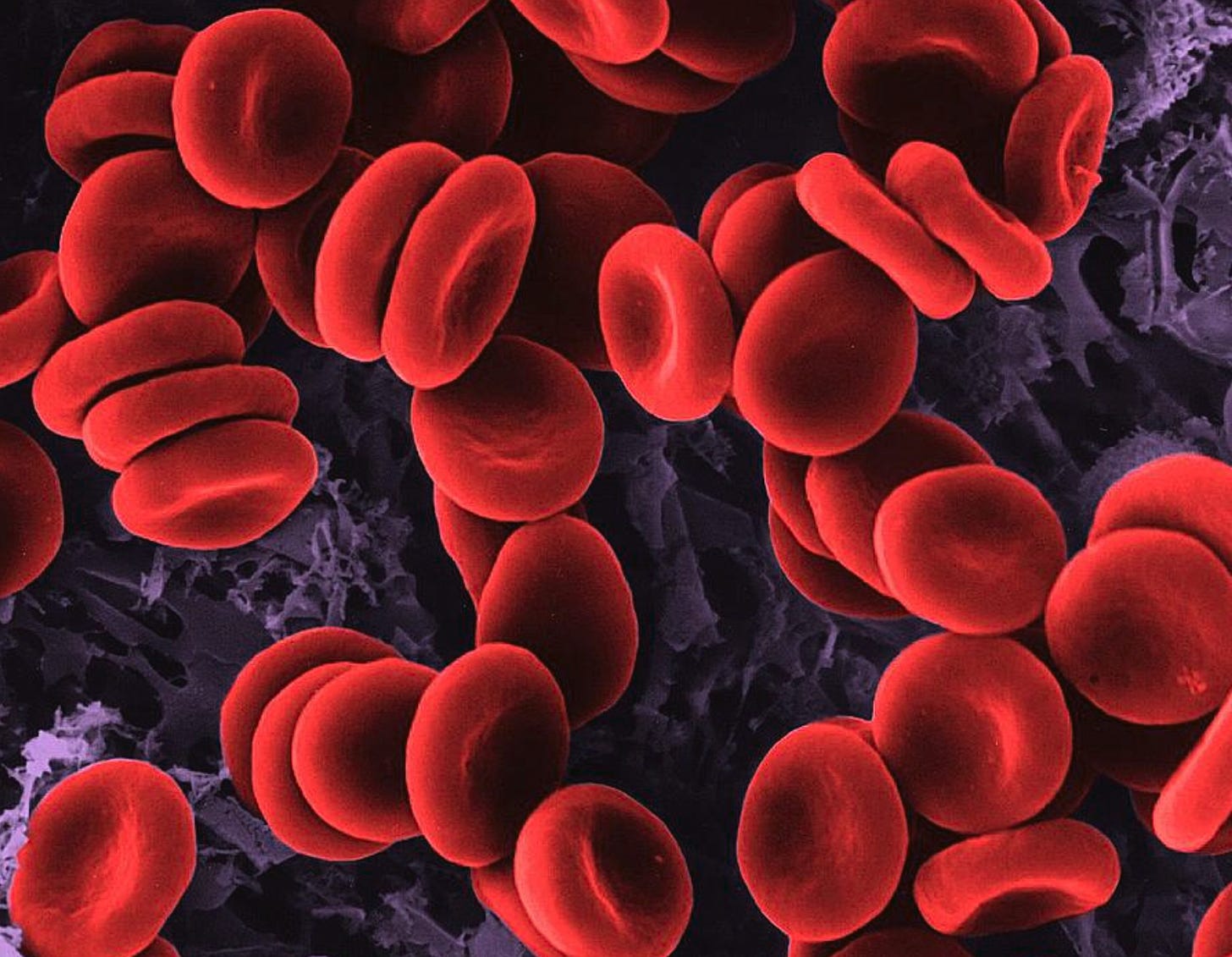
Malaria and COVID-19
SARS-nCoV-2 and the unicellular protozoan obligate parasite Plasmodium falciparum are the respective etiological agents of COVID-19 and malaria.[1],[2] They are very different entities whereby the former is a virus and latter is a single-celled parasite. However, these two entities have many similarities in terms of mechanisms of action and clinical manifestations.2
Plasmodium falciparum is a deadly species of Plasmodium that causes malaria in humans. It does its damage by infecting red blood cells (RBCs) and causes extensive changes in these cells which can ultimately result in hemolysis (rupture of the RBC).3 Plasmodium falciparum can enter RBCs via the receptor Cluster of Differentiation 147 (CD147).4 5 6 Once inside RBCs, Plasmodium falciparum feasts on hemoglobin. Subsequently, hemolysis causes a reduction in oxygen supply to all the tissues and organs in the body that require oxygen, which is literally all of the tissues and organs.
No oxygen supply = death.
Interestingly, SARS-nCoV-2 has also been shown to infect cells via CD147.7 8 SARS-nCoV-2 has also been associated with disruption of hemoglobin levels.9 The mechanism of action may involve the ability of SARS-nCoV-2 to attack the 1-beta chain of hemoglobin to capture its porphyrin and subsequently inhibit heme metabolism.10
The disease induced by Plasmodium falciparum, Malaria, is curable using (Hydroxy)chloroquine.11 12 13
“Antimalarial drugs such as chloroquine and possibly artemisinin inhibit hemoglobin detoxification by Plasmodium, underscoring the importance of this process for malarial viability”.14 15 Interestingly, studies have shown that hydroxychloroquine is also effective in the context of SARS-nCoV-2 and COVID-19?16[3])
Amyloid fibrils are insoluble protein nanofibers that spontaneously accumulate, or self-assemble, to form amyloid plaques and disease.17 Amyloid fibrils have been shown to develop from hemolysis of hemoglobin.18 Since Plasmodium falciparum can use CD147 to enter erythrocytes to disassemble hemoglobin to induce a disease state, then it is not only plausible, but likely, that amyloid plaque formation would ensue upon abundant hemolysis. It has been shown, in fact, that amyloidogenic peptides encoded in the Plasmodium organism play a role in malaria pathology.19
Recent publications reveal that the spike protein contains amyloidogenic peptides implicated in neurodegeneration.20 21 Since SARS-nCoV-2 can also use the CD147 receptor to enter erythrocytes22 23 to ‘disrupt’ hemoglobin, and has been evidenced to attack the beta chains of hemoglobin, does this also lend to induction of disease and generation of amyloid plaques via this mechanism of action?24 [4]
In this case, the pathology of COVID-19 would involve amyloid plaque formation based both on the amount of protein produced/present and the amount of damage to the red blood cells.
Amyloid fibrils have been shown to develop from hemolysis of hemoglobin contributing to thrombosis risk in severe COVID-19.25 Fibrin clots in individuals with ARDS associated with COVID-19 versus influenza, have been shown to be much denser. The reason for this was a reduced ability to break up clots (fibrinolysis) in the ARDS associated with COVID-19.
Dysregulation in RBC function in the context of SARS-nCoV-2 has been shown in recent publications where Heinz bodies were discovered in 14 individuals with severe COVID-19.26 27
The question now becomes, since the spike protein appears to induce hemolysis and amyloid plaque formation in the context of COVID-19, then what of the COVID-19 spike-based injectable products? This spike protein manufactured for use in the Pfizer and Moderna products, use the SARS-nCoV-2 spike as a template.[5]
Identification of Heinz bodies following COVID-19 injection could provide evidence of spike-induced RBC damage. Thus, in addition to measuring D-dimer levels, Heinz bodies and Lewy bodies[6] should be sought out as well in individuals with suspected COVID-19 injection-injuries. There is evidence of hemolysis in the context of the COIVD-19 injections,28 but more is required to provide proof.
Hypothesis: The SARS-nCoV-2 virus infects the RBCs using the spike protein via the CD147 receptor on RBCs causing hemolysis. This causes the release of massive amounts of hemoglobin. The spike protein, due to its amyloidogenic peptides, triggers mis-folding of the hemoglobin into amyloid fibrils causing subsequent systemic blood clots. If this pathology is spike protein induced, then there’s no reason not to implicate the spike protein arising from the COVID-19 injections in systemic micro-clotting observed clinically.
Question: Is SARS-nCoV-2-associated systemic micro-clotting due to spike protein-induced hemolysis resulting in amyloid plaque formation?
My opinion is YES.
[1] https://en.wikipedia.org/wiki/COVID-19
[2] https://en.wikipedia.org/wiki/Malaria
[3] https://covid19criticalcare.com/covid-19-protocols/i-recover-long-covid-treatment/
[4] https://news.cuanschutz.edu/news-stories/attack-on-red-blood-cells-a-prime-suspect-in-covids-debilitating-effects
[5] WHO-2019-nCoV-vaccines-SAGE_recommendation-BNT162b2-background-2021.1-eng.pdf. Page 3.
[6] https://en.wikipedia.org/wiki/Lewy_body
Mendonça MM, da Cruz KR, Pinheiro DDS, et al. Dysregulation in erythrocyte dynamics caused by SARS-CoV-2 infection: possible role in shuffling the homeostatic puzzle during COVID-19. Hematol Transfus Cell Ther. 2022;44(2):235-245. doi:10.1016/j.htct.2022.01.005.
Hussein MIH, Albashir AAD, Elawad OAMA, Homeida A. Malaria and COVID-19: unmasking their ties. Malar J. 2020;19(1):457. Published 2020 Dec 23. doi:10.1186/s12936-020-03541-w.
Mohandas N, An X. Malaria and human red blood cells. Med Microbiol Immunol. 2012 Nov;201(4):593-8. doi: 10.1007/s00430-012-0272-z. Epub 2012 Sep 11. PMID: 22965173; PMCID: PMC3699179.
Wang, Ke & Chen, Wei & Zhou, Yu-Sen & Lian, Jian-Qi & Zhang, Zheng & Du, Peng & Gong, Li & Zhang, Yang & Cui, Hongyong & Geng, Jie-Jie & Wang, Bin & Sun, Xiu-Xuan & Wang, Chun-Fu & Yang, Xu & Lin, Peng & Deng, Yong-Qiang & Wei, Ding & Yang, Xiang-Min & Zhu, Yu-Meng. (2020). SARS-CoV-2 invades host cells via a novel route: CD147-spike protein. 10.1101/2020.03.14.988345.
Pretini Virginia, Koenen Mischa H., Kaestner Lars, Fens Marcel H. A. M., Schiffelers Raymond M., Bartels Marije, Van Wijk Richard. Red Blood Cells: Chasing Interactions. Front. Physiol., 31 July 2019. Sec. Red Blood Cell Physiology. https://doi.org/10.3389/fphys.2019.00945.
Crosnier, C. et al. Basigin is a receptor essential for erythrocyte invasion by Plasmodium falciparum. Nature 480, 534-537 (2011).
Wang, K., Chen, W., Zhang, Z., Deng, Y., Lian, J. Q., Du, P., Wei, D., Zhang, Y., Sun, X. X., Gong, L., Yang, X., He, L., Zhang, L., Yang, Z., Geng, J. J., Chen, R., Zhang, H., Wang, B., Zhu, Y. M., Nan, G., … Chen, Z. N. (2020). CD147-spike protein is a novel route for SARS-CoV-2 infection to host cells. Signal transduction and targeted therapy, 5(1), 283. https://doi.org/10.1038/s41392-020-00426-x.
Ulrich H, Pillat MM. CD147 as a Target for COVID-19 Treatment: Suggested Effects of Azithromycin and Stem Cell Engagement. Stem Cell Rev Rep. 2020 Jun;16(3):434-440. doi: 10.1007/s12015-020-09976-7. PMID: 32307653; PMCID: PMC7167302.
Lippi G, Mattiuzzi C. Hemoglobin value may be decreased in patients with severe coronavirus disease 2019. Hematol Transfus Cell Ther. 2020 Apr-Jun;42(2):116-117. doi: 10.1016/j.htct.2020.03.001. Epub 2020 Apr 2. PMID: 32284281; PMCID: PMC7128154.
Liu and Li. COVID-19: Attacks the 1-Beta Chain of Hemoglobin and Captures the Porphyrin to Inhibit Heme Metabolism. https://chemrxiv.org/engage/api-gateway/chemrxiv/assets/orp/resource/item/60c74fa50f50db305139743d/original/covid-19-attacks-the-1-beta-chain-of-hemoglobin-and-captures-the-porphyrin-to-inhibit-human-heme-metabolism.pdf
Coy D. Fitch and Natrice V. Russell. Accelerated Denaturation of Hemoglobin and the Antimalarial Action of Chloroquine. ASM Journal. Antimicrobial Agents and Chemotherapy. Vol. 50, No. 7.
White NJ. The treatment of malaria. N Engl J Med. 1996;335(11):800-806. doi:10.1056/NEJM199609123351107.
Slater AF. Chloroquine: mechanism of drug action and resistance in Plasmodium falciparum. Pharmacol Ther. 1993;57(2-3):203-235. doi:10.1016/0163-7258(93)90056-j.
Ziegler J, Linck R, Wright DW (2001) Heme Aggregation inhibitors: antimalarial drugs targeting an essential biomineralization process. Curr Med Chem 8: 171–189.
Klonis N, Crespo-Ortiz MP, Bottova I, Abu-Bakar N, Kenny S, et al. (2011) Artemisinin activity against Plasmodium falciparum requires hemoglobin uptake and digestion. Proc Natl Acad Sci U S A 108: 11405–11410.
de Reus YA, Hagedoorn P, Sturkenboom MGG, et al. Tolerability and pharmacokinetic evaluation of inhaled dry powder hydroxychloroquine in healthy volunteers. PLoS One. 2022;17(8):e0272034. Published 2022 Aug 5. doi:10.1371/journal.pone.0272034.
R. Wetzel, in Encyclopedia of Biological Chemistry (Second Edition), 2013.
Jayawardena N, Kaur M, Nair S, Malmstrom J, Goldstone D, Negron L, Gerrard JA, Domigan LJ. Amyloid Fibrils from Hemoglobin. Biomolecules. 2017 Apr 11;7(2):37. doi: 10.3390/biom7020037. PMID: 28398221; PMCID: PMC5485726.
Moles E, Valle-Delgado JJ, Urbán P, et al. Possible roles of amyloids in malaria pathophysiology. Future Sci OA. 2015;1(2):FSO43. Published 2015 Sep 1. doi:10.4155/fso.15.43.
Charnley, M., Islam, S., Bindra, G.K. et al. Neurotoxic amyloidogenic peptides in the proteome of SARS-COV2: potential implications for neurological symptoms in COVID-19. Nat Commun 13, 3387 (2022). https://doi.org/10.1038/s41467-022-30932-1.
Tetz G, Tetz V. Prion-like Domains in Spike Protein of SARS-CoV-2 Differ across Its Variants and Enable Changes in Affinity to ACE2. Microorganisms. 2022; 10(2):280. https://doi.org/10.3390/microorganisms10020280.
Wang, K., Chen, W., Zhang, Z., Deng, Y., Lian, J. Q., Du, P., Wei, D., Zhang, Y., Sun, X. X., Gong, L., Yang, X., He, L., Zhang, L., Yang, Z., Geng, J. J., Chen, R., Zhang, H., Wang, B., Zhu, Y. M., Nan, G., … Chen, Z. N. (2020). CD147-spike protein is a novel route for SARS-CoV-2 infection to host cells. Signal transduction and targeted therapy, 5(1), 283. https://doi.org/10.1038/s41392-020-00426-x.
Ulrich H, Pillat MM. CD147 as a Target for COVID-19 Treatment: Suggested Effects of Azithromycin and Stem Cell Engagement. Stem Cell Rev Rep. 2020 Jun;16(3):434-440. doi: 10.1007/s12015-020-09976-7. PMID: 32307653; PMCID: PMC7167302.
Mendonça MM, da Cruz KR, Dos Santos Silva FC, Fontes MAP, Xavier CH. Are hemoglobin-derived peptides involved in the neuropsychiatric symptoms caused by SARS-CoV-2 infection? [published online ahead of print, 2022 Jul 27]. Braz J Psychiatry. 2022;10.47626/1516-4446-2021-2339. doi:10.47626/1516-4446-2021-2339.
Wygrecka M.et al; Altered fibrin clot structure and dysregulated fibrinolysis contribute to thrombosis risk in severe COVID-19. Blood Adv 2022; 6 (3): 1074–1087. doi: https://doi.org/10.1182/bloodadvances.2021004816.
Mendonça MM, da Cruz KR, Pinheiro DDS, et al. Dysregulation in erythrocyte dynamics caused by SARS-CoV-2 infection: possible role in shuffling the homeostatic puzzle during COVID-19. Hematol Transfus Cell Ther. 2022;44(2):235-245. doi:10.1016/j.htct.2022.01.005.
Perrin and Gérard. Heinz bodies in COVID-19. https://onlinelibrary.wiley.com/doi/epdf/10.1111/ijlh.13926
Gloria F. Gerber, Xuan Yuan, Jia Yu, Benjamin A. Y. Cher, Evan M. Braunstein, Shruti Chaturvedi, Robert A. Brodsky; COVID-19 vaccines induce severe hemolysis in paroxysmal nocturnal hemoglobinuria. Blood 2021; 137 (26): 3670–3673. doi: https://doi.org/10.1182/blood.2021011548.




Thank you Dr. Rose for your work re: amyloid. I had a feeling this might be a consequence of the jabs/SP. This is information is invaluable to those of us who are concerned about potential amyloidosis. A thousand thank yous....
Luc Montagnier said the virus contained HIV and a “malaria germ” and I keep wondering what that means