Remember I thought myocarditis might actually be cardiac amyloidosis?
In many cases, it might well be. A case study confirms.
Thank you to Walnut for bringing this to my attention today. What amazing new friends and colleagues I have.
This is a case study (CASE 16643) from Cairo presented by EuroRad entitled: “Acute presentation of a hidden cardiac amyloidosis and microvascular occlusion revealed by CMR”. It was actually published 06.03.2020 and I am sure many more cases like this have been found around the globe.
I have previously Substacked my thoughts on this subject matter:
This case study is the story of a 21-year old male who was initially diagnosed with acute myocarditis and referred for MRI, His diagnosis changed to cardiac amyloidosis following cardiovascular magnetic resonance and follow-up tests.
The take home message of the case study write-up:
Our take home message: don’t exclude amyloid in acute chest pain with patent coronaries even if the age is not typical. It is highly recommended to use cardiac MRI in case of acute chest pain with patent epicardial coronaries for the diagnostic and prognostic values.
Here’s the full write-up.
CLINICAL HISTORY
A 21-year-old male patient with acute chest pain, ECG revealed sinus tachycardia, cardiac enzymes were mildly elevated.
Echocardiography revealed minimal pericardial effusion. CT revealed patent
coronaries, no vasculitis or abnormal coronary position. There was upper lung
mild ground glassing reported as pneumonia. Patient was diagnosed as acute
myocarditis and referred for MRI.
IMAGING FINDINGS
There was left ventricular symmetrical concentric hypertrophy, left ventricular myocardial mass index measures 118 gm/m2 and maximal myocardial thickness measures 17 mm. There was a reduced left ventricular diastolic filling with EDVI 61 ml/m2 and pseudo-normalised systolic function with EF 61%.
The striking finding was the diffuse myocardial oedema in myocardium as regard increased myocardial signal in the T2 fat-suppression images more than 2 SD compared to the skeletal muscles.
There was diffuse circumferential subendocardial resting perfusion defects suggesting diffuse microangiopathy in correlation with the patent epicardial coronaries.
The TI scout revealed earlier drop of myocardial signal in comparison to blood pool which raised the suspicion of amyloidosis, the delayed gadolinium enhancement revealed multiple subendocardial foci of microvascular occlusion (MVO) and failure of myocardial nulling even in high inversion time values (700 msec) involving all myocardium which confirmed the diagnosis of cardiac amyloidosis and on top acute microvascular occlusion.
DISCUSSION
There are two types of cardiac amyloidosis: abnormal transthyretin (ATTR) amyloidosis and light-chain (AL) amyloidosis.
Cardiac amyloidosis leads to restrictive cardiomyopathy, ECG changes as decreased QRS voltage, aggressive arrhythmias up to sudden cardiac death more in AL in comparison to ATTR amyloidosis.
Chest pain secondary to myocardial ischaemia is a presentation of cardiac amyloidosis and is attributed to the deposition of protein molecules in the coronary microvasculature which can spare the epicardial coronaries. With reported similar cases with MVO.
Diagnostic pathway of cardiac amyloidosis includes: echocardiography cardiovascular magnetic resonance (CMR) which also gives a prognostic value, 99m Technetium pyrophosphate (Tc99m-PYP) and 99m Technetium 3,3-diphosphono-1,2-propanodicarboxylic acid (DPD), laboratory work-up and biopsy.
T1 mapping significantly increases native T1 values even if no cardiac involvement was suspected by clinical and echocardiographic criteria. There was no overlap between myocardial T1 values in patients with definite cardiac involvement of amyloidosis and normal control subjects. T1 values in these patients elevated in the range of 5–10 standard deviations above the normal mean, extra-cellular volume (ECV) is markedly increased with high degree of sensitivity and specificity. It is also correlated to the ejection fraction.
18F-florbetaben-PET/CT is the most recent method in AL-amyloidosis followed by ATTR-amyloidosis.
Young age of cardiac amyloid involvement is more with ATTR type.
Teaching point
Although age was not typical in our patient, the cardiac MRI pattern was highly suggestive of cardiac amyloidosis, considering the diffuse subendocardial delayed enhancement, failure of myocardial nulling even in high TI values, and circumferential myocardial thickening. In addition to the poor prognostic criteria in the form of three-vessel acute microangiopathic occlusion (no reflow phenomena) and resting perfusion defect.




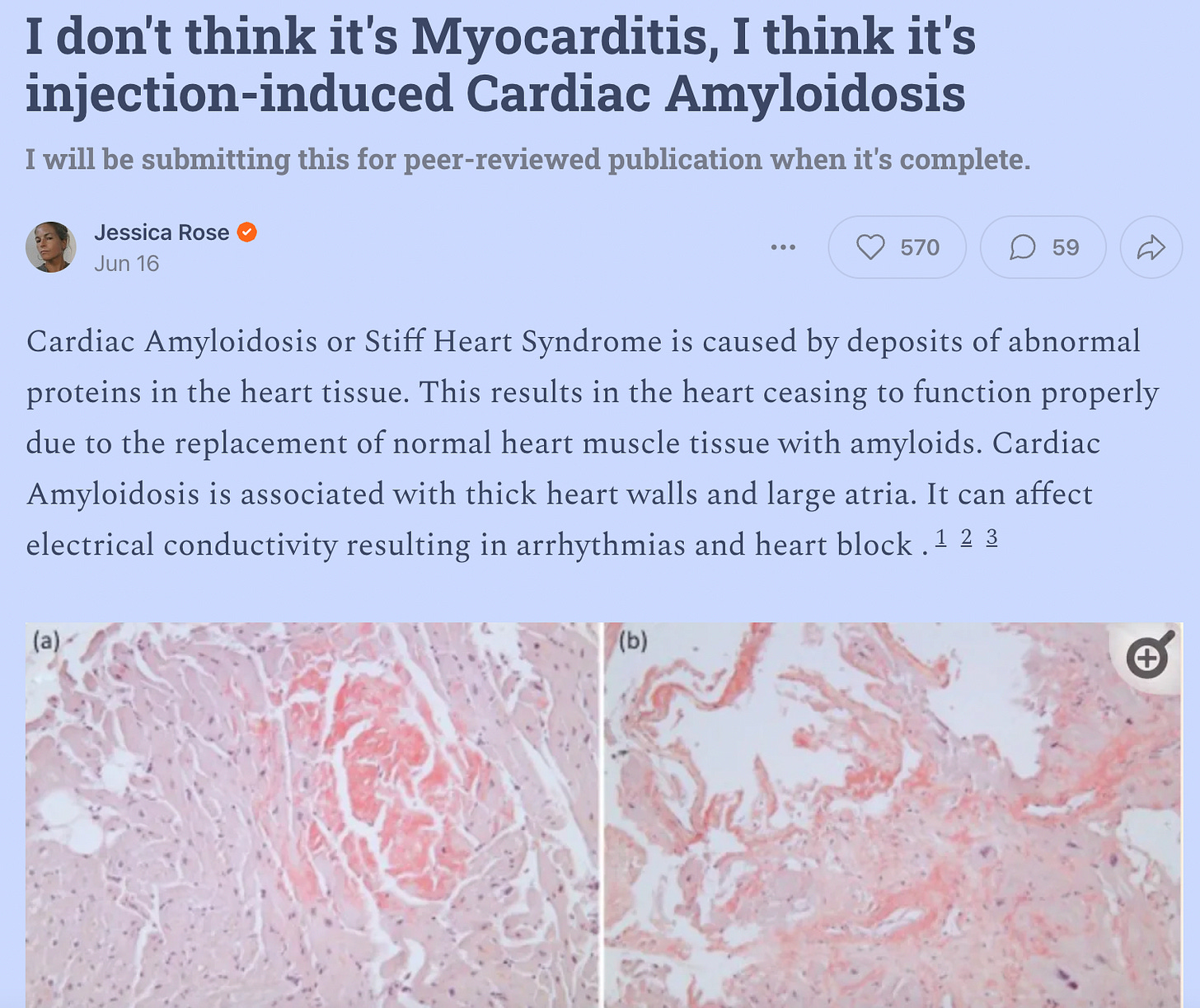

The conclusion you reach about cardiac amyloidosis makes sense to me, a non-medically trained reader. But the elephant in the room that struck me was amyloid protein as being the substance detected in the heart capillaries.
It’s an insoluble protein. Is that not the description of the substance Mr. Hirschhorn and other morticians extracted from the blood vessels of the deceased injection victims?
This obvious linkage between the characteristics of this substance synthesized from components of blood seems to suggest a method has been created by the spike protein injections to create a durable, insoluble substance that’s operative at macro as well as micro circulatory levels.
Its manifestation in the heart tissue mocks the symptoms of myocardial damage, and thus leads many to the mistaken conclusion it’s myocarditis. Are we talking about the same rubbery stuff?
THANK you so much. I am certain this is what killed my Mom. She was cremated but had tissues and blood saved in the event there was a new study or tests for some new possible cause (LIKE THIS!).
Is there a way you know to test her blood for this amyloidosis? And IF IT IS amyloidosis does it usually track those fibrous clots would be in the veins/arteries? And since I am vaccine injured/hijacked by Murderna, I am wondering how to check for these while I am still living!! Have you heard of anyone removing them pre-emptively?
Thank you!